Introduction:
Belamaf (GSK2857916), a B-cell maturation antigen-targeting antibody-drug conjugate (ADC), demonstrated deep and durable responses with a manageable safety profile as a single agent in patients with heavily pretreated RRMM in the pivotal DREAMM-2 study (NCT03525678; Lonial ASCO 2020 Poster 436). Health-related quality of life (HRQoL) was evaluated via the cancer-specific European Organisation for Research and Treatment of Cancer quality of life questionnaire (EORTC-QLQ-C30; Popat EHA 2020 Poster EP1746), a PRO used extensively in oncology/MM studies to evaluate symptoms, functioning, and QoL. The EORTC-QLQ-MY20 module was used to assess MM symptoms. Corneal events are expected during belamaf treatment, as with other monomethyl auristatin F-containing ADCs, so two ophthalmic vision-related PRO questionnaires (National Eye Institute Visual Function Questionnaire-25 item [NEI-VFQ-25] and Ocular Surface Disease Index [OSDI]) were used to characterize the impact of corneal events on patient symptoms and visual function. Meaningful within-patient changes in OSDI and NEI-VFQ-25 scores were estimated to better interpret outcomes in this population (Eliason ISPOR 2020). We report here the results of the PRO analyses in DREAMM-2 according to measures used in the trial.
Methods:
In DREAMM-2, patients who received single-agent belamaf (2.5 or 3.4 mg/kg, every 3 weeks [Q3W]) completed PRO questionnaires electronically at baseline and Q3W during treatment. Group-level, mean change from baseline over time was evaluated on EORTC domains. We also evaluated the percentage of patients with ≥10-point meaningful change threshold for improvement (Osoba J Clin Oncol 1998) on EORTC domains over time. Meaningful change thresholds in ocular PROs measuring treatment-related corneal events were estimated using recommended anchor and distribution-based methods, with 12.5-16.6 points estimated as meaningful in this population, depending on the domain (Eliason ISPOR EU 2020). We report results of the PRO data analysis for patients in the 2.5-mg/kg group selected for clinical development.
Results:
At Weeks 7 and 13, 46% (21/46) and 41% (12/29) of patients who completed PROs improved ≥10 points in the EORTC-QLQ-C30 Fatigue domain score, respectively; 30% (14/46) and 31% (9/29) improved their General Pain domain score. For EORTC-QLQ-C30, there were trends toward improvement in Fatigue at some time points on treatment; Global Health Status (GHS)/QoL, Role Functioning, and Physical Functioning domain scores remained relatively stable. The EORTC-QLQ-MY20 Disease Symptoms domain score (representing pain in different locations) showed a general trend toward improvement over time, with improvements of ≥10 points at Weeks 7 and 13 for 38% (17/45) and 29% (8/28) of participants.
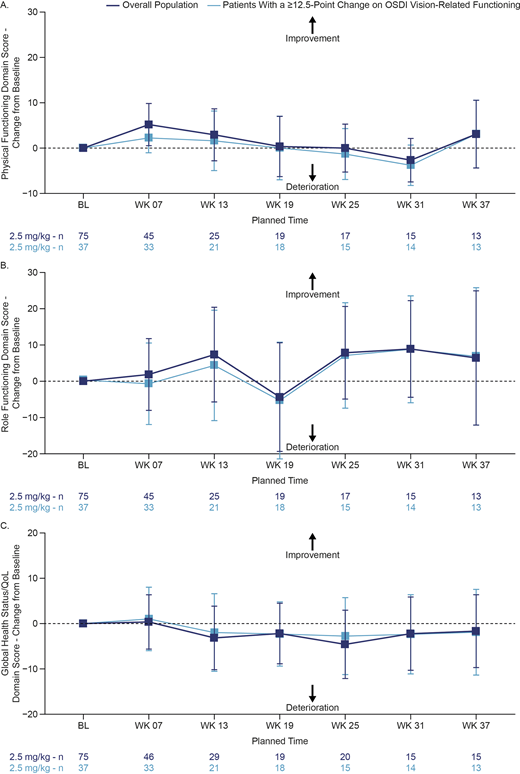
Ocular PRO data were available for 95% (92/97) of patients. Based on the OSDI vision-related functioning domain, a total of 49.5% of patients experienced a ≥12.5-point worsening from baseline (median time to worsening: 44 days). Meaningful improvement of these changes (based on defined 12.5-point thresholds) from worst severity post baseline was seen in 72% of patients (median time to improvement: 24 days). Importantly, even among patients with meaningful worsening in visual functioning, patient-reported QoL/GHS, Physical Functioning, and Role Functioning domains of the EORTC-QLQ-C30 remained stable while on treatment (Figure).
Conclusions:
Disease symptoms, functioning, and QoL did not worsen over time in these heavily pretreated patients receiving belamaf in DREAMM-2. Patients showed a general improvement in fatigue, which is often a difficult-to-manage symptom for patients with RRMM. Group-level, meaningful worsening in vision-related PRO domains was observed, which improved in the majority of patients. Despite ocular symptoms, even in patients with meaningful worsening, EORTC-QLQ-C30 data suggest that overall HRQoL and patient functioning remained stable while on treatment. These PRO results demonstrate a balance between overall QoL/functioning and vision-related impacts that, together with its clinical efficacy, supports the use of belamaf in the treatment of patients with RRMM.
Funding: GSK (study 205678); drug linker technology licensed from Seattle Genetics; mAb produced using POTELLIGENT Technology licensed from BioWa.
Popat:GSK: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Celgene: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other: Travel support, Research Funding; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); AbbVie: Consultancy, Honoraria. Lonial:Novartis: Consultancy, Honoraria, Other: Personal fees; Takeda: Consultancy, Other: Personal fees, Research Funding; Amgen: Consultancy, Honoraria, Other: Personal fees; Sanofi: Consultancy; Karyopharm: Consultancy; GSK: Consultancy, Honoraria, Other: Personal fees; Abbvie: Consultancy; Merck: Consultancy, Honoraria, Other: Personal fees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; JUNO Therapeutics: Consultancy; Millennium: Consultancy, Honoraria; Onyx: Honoraria; Genentech: Consultancy; Janssen: Consultancy, Honoraria, Other: Personal fees, Research Funding; BMS: Consultancy, Honoraria, Other: Personal fees, Research Funding. Voorhees:Adaptive Biotechnologies: Other: Personal fees; Levine Cancer Institute, Atrium Health: Current Employment; TeneoBio: Other: Personal fees; Oncopeptides: Other: Personal fees; Novartis: Other: Personal fees; Janssen: Other: Personal fees; Celgene: Other: Personal fees; Bristol-Myers Squibb: Other: Personal fees. Degli Esposti:Moorfields Eye Hospital: Current Employment; GlaxoSmithKline: Consultancy, Honoraria. Gupta:GlaxoSmithKline: Current Employment, Current equity holder in publicly-traded company; Novartis: Current equity holder in publicly-traded company. Opalinska:GSK: Current Employment, Current equity holder in publicly-traded company. Sapra:GSK: Current Employment, Current equity holder in publicly-traded company. Gorsh:GSK: Current Employment, Current equity holder in publicly-traded company. He:GSK: Current Employment, Current equity holder in publicly-traded company. Kleinman:Triphase Accelerator U.S Corporation: Consultancy; ONL Therapeutics, Inc: Consultancy; Revolution Medicines, Inc: Consultancy; Editas Medicine, Inc: Consultancy; Cleave Therapeutics, Inc: Consultancy; Coherus Biosciences, Inc: Consultancy; Synergy Research Inc: Consultancy; GSK: Consultancy; Eyeon Therapeutics, LLC.: Current equity holder in private company; Zenith Epigenetics Ltd: Consultancy. Schaumberg:Evidera, Inc: Current Employment; GSK: Consultancy; Novaliq: Consultancy; SilkTech: Consultancy; University of Utah School of Medicine: Current Employment. Loubert:Modus Outcomes: Current Employment. Meunier:Modus Outcomes: Current Employment. Regnault:Modus Outcomes: Current Employment. Eliason:GSK: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal